PROPERTIES OF LIGHT THAT ARE IMPORTANT FOR PHOTOSYNTHESIS
A review of the general lighting requirements for photosynthesis reveals that four aspects of light are important: irradiance, quality, timing and duration. These properties of light affect photosynthesis by providing the energy that drives carbon assimilation as well as by exerting control over physiology, structure and morphology of plants. Irradiance, expressed as energy flux, W m-2, or photon irradiance, µmol m-2 s-1, determines the rate at which energy is being delivered to the photosynthetic reaction centers. Spectral quality, the wavelength composition of light, is important because photons differ in their probability of being absorbed by the light harvesting complex and hence their ability to drive carbon assimilation. Also the various light receptors for light-mediated regulation of plant form and physiology have characteristic absorption spectra and hence photons differ in their effectiveness for eliciting responses. Duration is important because both carbon assimilation and regulation are affected by the total energy or integrated irradiance delivered during a given period. Many processes associated with photosynthesis are time-dependent, increasing or decreasing with duration. Timing is important because the effectiveness of light in the regulation of plant processes varies with the phase of the diurnal cycle as determined by the plant’s time-measuring mechanisms.
Physiologically Important Measures of Light
Photosynthetic photon flux or PPF (ex. µmol m-2 s-1), a combination of irradiance and spectral quality, is a measure of the photosynthetically active photon irradiance (PAR or photosynthetically active radiation defined as the irradiance between the wavelengths of 400 and 700 nm). PPF is the maximum energy available and only a small proportion of the photons actually are used to assimilate carbon.
Time course of diurnal PPF and integrated diurnal irradiance, combinations of irradiance and duration, have important effects on photosynthetic carbon metabolism and its regulation. Of particular importance is the rapidity with which the light begins. Maximum irradiance level and the duration of high irradiance are important aspects of the time course of diurnal PPF, potentially affecting the degree of photoinhibition or photoprotection, both of which can lower the efficiency of light use for photosynthesis. It is important that these aspects of irradiance generally be similar to those under which the plant developed.
Daytime spectral quality and end of day spectral quality are a combination of quality and timing. Not only does light drive photosynthesis but irradiance that extends beyond the range of 400 to 700 nm affects plant morphology, physiology, leaf display and chloroplast orientation. Properties of light that affect growth and morphology of the plant, in turn, can affect photosynthesis. Photosynthesis generates a positive feedback system in which photoassimilation of carbon contributes to the growth of leaves that absorb photons and assimilate carbon to contribute to further growth and so on. The compound interest aspect of the production and growth of leaves obviously is affected by plant properties such as leaf area and thickness, which are regulated by light.
Photoperiod, the duration and timing of the irradiance, has marked effects on plant physiology and morphology, including carbon allocation, root to shoot ratio and reproduction. A combination of duration and irradiance comprise the integrated diurnal light energy that determines both the total daily assimilation of carbon but also affects morphology and physiology of leaves.
The four properties of light, alone and in combination, are important to consider in the design and evaluation of performance of plant lighting systems. Responses to the various aspects of light are conditioned by adaptation, the genetically determined range of possible responses. For example, plants that are adapted to growing in the sun will have a certain maximum photosynthetic capacity which will be considerably higher than that of a shade plant. On the other hand, plants adapted to grow in shade will be able to survive at a lower photosynthesis rate. Within the range of the adaptive possibilities, plants undergo acclimation to actual conditions.
SPECTRAL QUALITY
Light Quality Affects Photochemical Reactions
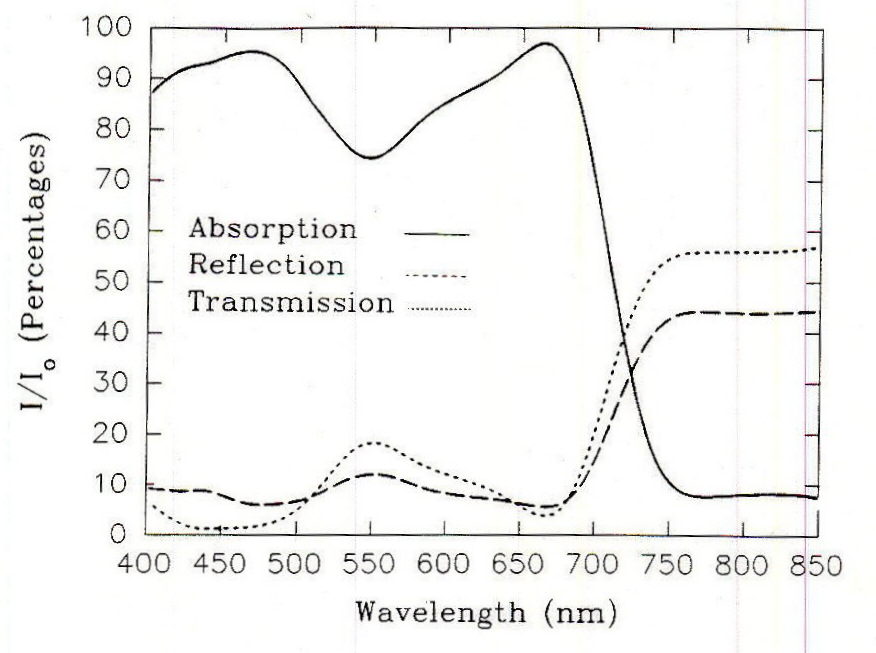
Fig. 1. Absorption, reflection and transmission of light by a typical soybean leaf. I/Iorefers to the radiation absorbed, transmitted or reflected relative to incident radiation at the same wavelength. From Kasperbauer, 1987.
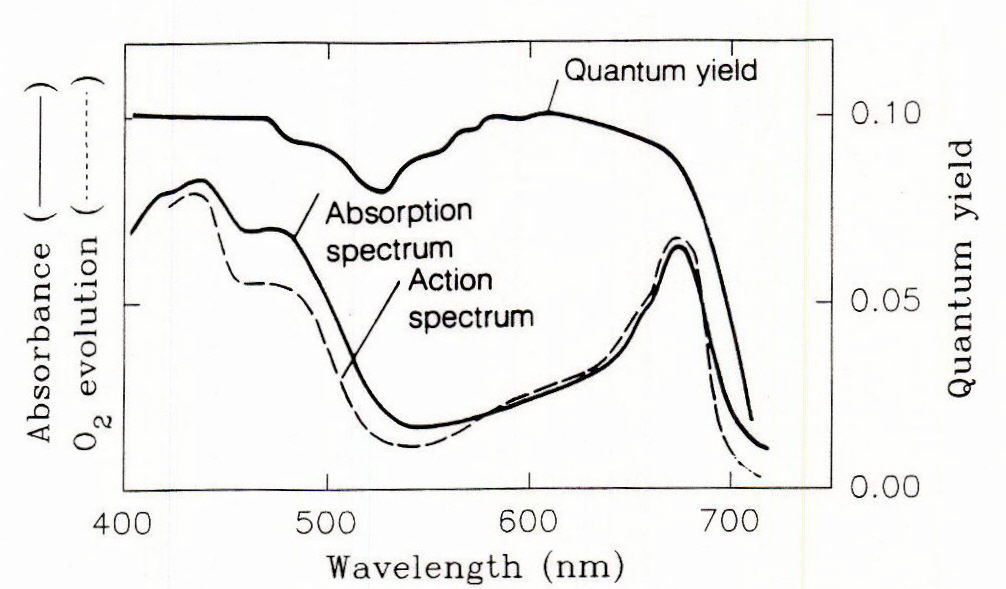
The types, amounts and structural configuration of the photosynthetic pigments present in the photosystems, along with chloroplast orientation, determine the extent to which the various photons are absorbed (Figure 1). The two major absorption peaks are related to the presence of chlorophylls and carotenoids arranged in photosystem antenna complexes and reaction centers. The general correspondence between the absorption and action spectra indicate that the photons that are absorbed are used with generally similar efficiency. Plants adapted to higher light generally have the capacity to develop antenna complexes capable of absorbing these higher irradiances efficiently and, within the range of possibilities, the leaves will acclimate to a specific irradiance range by developing complexes with a certain capacity. Examination of the quantum yield of photons reveals that the photosynthetic efficiency of photons is generally similar between 400 and 680 nm, with a rapid fall off above the latter wavelength (Figure 2). Photosynthesis using light absorbed at wavelengths between the chlorophyll absorption peaks has a slightly lower efficiency.
Fig. 2 Comparison of action spectrum and quantum yield for photosynthesis with the chloroplast absorption spectrum. Quantum yield of photosynthesis is the moles of carbon fixed per mole of photons absorbed. From Taiz and Zeiger, 1991.
The energy content of the region of PAR determines the availability of energy for driving photosynthesis while that in the band above 650 is particularly important in photomophogenic processes mediated by phytochromes. Quantum yield of photosynthesis under a given set of conditions depends on absorption of photons by pigments of the antenna complexes, use of the excitation energy transferred from the molecules of the antenna complex to drive photochemistry in the reaction centers, and use of this energy in carbon assimilation. It is important that there be a close match between the amount of light absorbed and the amount actually used to drive photosynthetic carbon assimilation. Environmental factors may result in dissipation of absorbed energy by various processes that result in a decrease in quantum yield (Krause and Weis, 1991; Demmig-Adams and Adams 1992; Ruban et al., 1993).
Light Quality Affects Plant Structure, Morphology and Physiology
Light quality affects morphology, biochemistry and physiology of plants through its action on phytochromes and other pigments associated with light-mediated regulation of processes (Lopez-Juez et al., 1990). Examples of physiological parameters that affect photosynthesis are shown in Table 1.
TABLE 1 Effect of Light Quality on Physiological Parameters for Leaves of Wild Type and Mutant Cucumber. Parameters shown for wild type and long hypocotyl mutant after 20 days in daily photoperiods of 14 h white fluorescent with (+FR) or without (-FR) 20 min of far red light at the end of the day. From Lopez-Juez et al., 1990.
| Parameter (units) | Wild type
+FR |
Wild type
+FR |
lh mutant
-FR |
lh mutant
-FR |
|
Chlorophyll content (mg g-1 fr wt) |
2.16 |
2.07 |
1.63 |
1.69 |
|
Chlorophyll content (µg cm-2) |
43.3 |
36.3 |
21.1 |
22.7 |
|
Chlorophyll a/b ratio
|
2.7 |
2.6 |
2.6 |
2.5 |
| Total carotenoid
(mg g-1 fr wt) |
0.28 |
0.27 |
0.22 |
0.21 |
|
Soluble protein (mg g-1 fr wt) |
9.3 |
7.6 |
7.4 |
7.2 |
|
Photosynthesis rate (mg CO2 m-2 s-1) (µg CO2 mg Chl-1 s-1) |
0.29 0.66 |
0.18 0.48 |
0.06 0.26
|
0.08 0.36 |
The far red light results in less chlorophyll per area and a lower photosynthesis rate per area, effects that can be explained by the fact that far red light given at the end of the day results in larger but thinner leaves. These leaves also seem to have a somewhat lower efficiency of photosynthesis per unit of chlorophyll. The fact that leaf morphology and physiology can be adjusted by a short period of light that has a specific spectral quality under come conditions may offer an efficient alternative to exposing plants to light with an energy-expensive spectral balance throughout a whole day.
DIURNAL TIME COURSE OF IRRADIANCE
In the Field Irradiance Changes Gradually Throughout the Day
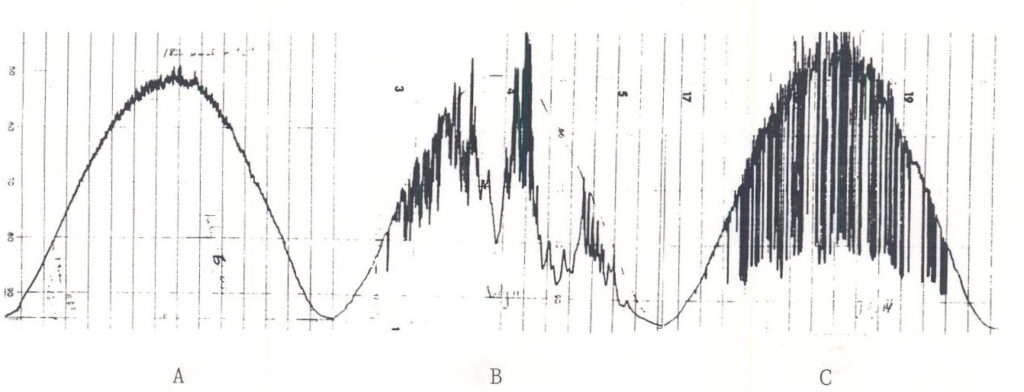
Irradiance is a major factor influencing photosynthesis rate. The amount of sunlight striking a unit area of the earth at any time in the course of a day is a direct function of the sine of the angle that the sun makes with the earth’s surface. On a clear day, irradiance level on a horizontal surface generally follows the sine of the sun’s angle with earth’s surface (Figure 3). Even when there are clouds irradiance gradually increases and decreases over the course of a day.
Fig. 3. Time courses of irradiance during July. A. Clear day. B. Partly cloudy day. C. Partly sunny day.
The light available to leaves for photosynthesis depends both on the time course of diurnal irradiance and on factors, such as leaf orientation, that affect the interception of the incident light. Evolution of photosynthesizing organisms under cyclic diurnal irradiance has equipped plants to regulate the photosynthetic process in ways that allow carbon to be assimilated efficiently over the wide range of diurnal cycle irradiance. A particularly important aspect of this diurnal time course is the fact that irradiance begins slowly, generally matching the time constants of processes related to photosynthesis. For instance stomatal opening and induction of photosynthetic carbon assimilation by the Calvin cycle generally have time constants on the order of minutes. Induction of photosynthesis involves building the concentration of metabolite pools and enzyme activities associated with photosynthetic carbon assimilation. Beginning a photoperiod with rapid, practically instantaneous irradiance can lead to light and water stress (Geiger et al. 1994).
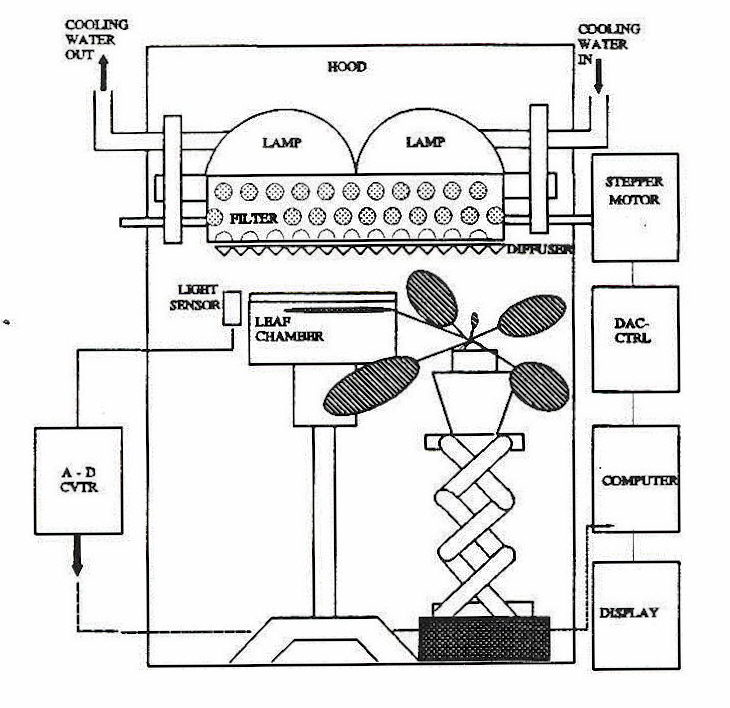
Because plants have become adapted to gradually changing daily irradiance, it is advantageous to study regulation of photosynthesis and carbon metabolism in the context of the diurnal light cycle. In particular, timing aspects of carbon assimilation regulation can be analyzed more effectively because the gradual changes in irradiance allow us to observe the step by step progress of the daily acclimation process. A number of researchers used this approach to elucidate mechanisms involved in the regulation of photosynthesis in nature (references in Geiger and Servaites, 1994a). Recently our laboratory has undertaken as series of studies of the regulation of photosynthesis (Geiger et al., 1991, Servaites et al, 1989a, 1989b, 1991) with the help of an apparatus that simulates the gradually changing irradiance of a natural diurnal light regime (Figure 4).
Fig. 4. Apparatus for regulating irradiance to simulate the irradiance under a natural light regime. The light level is sensed and converted to digital input (A-D CVTR) for processing by a computer. A signal is sent to a controller (ADC-CTR) which then directs the motor to transport the neutral-density film to produce the required irradiance. From Geiger et al., 1991.
Physiological Aspects of Gradually Changing Diurnal Irradiance
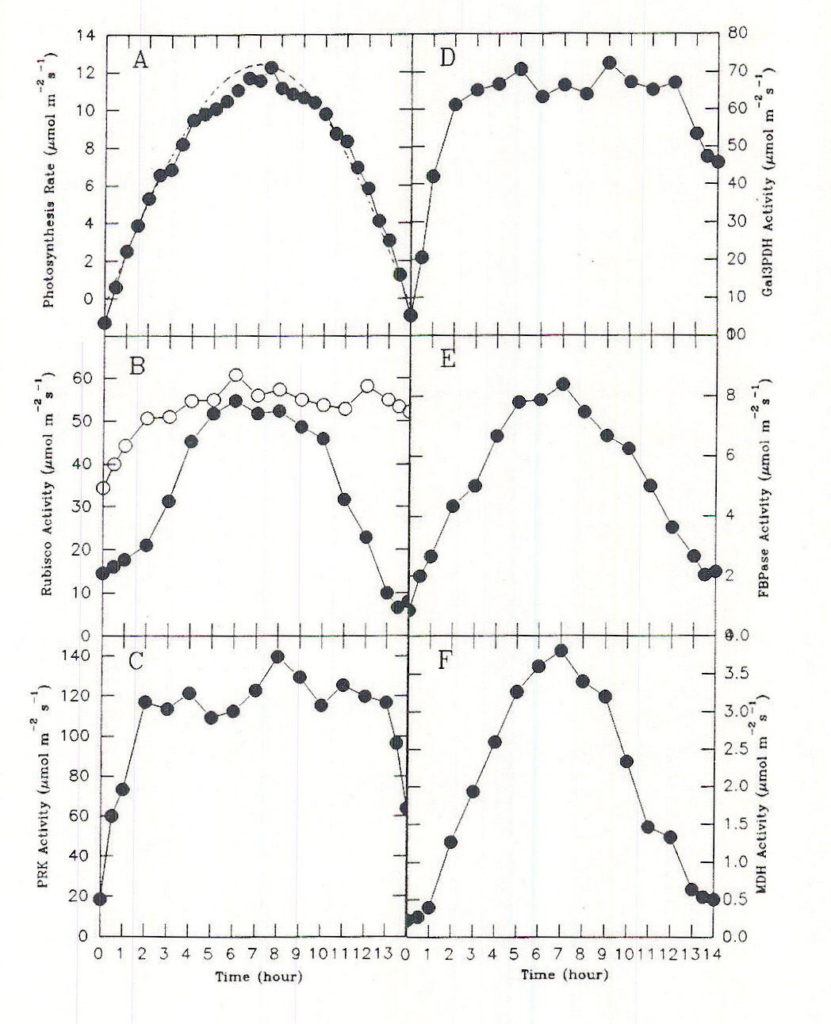
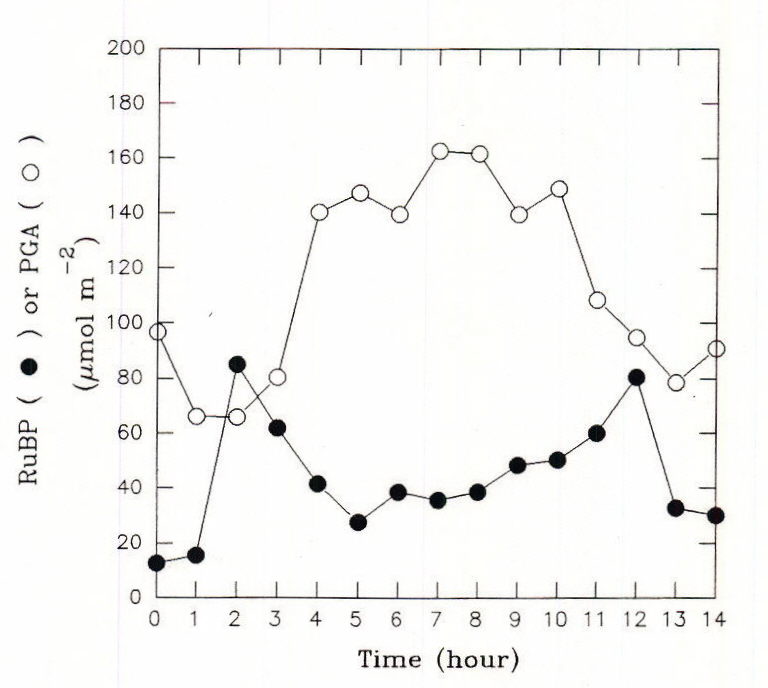
To keep pace with changing PPF, flux through the carbon assimilation cycle changes over a wide range during the course of a single day. Unless prevented by some overriding factor, diurnal regulation enables the photosynthesis rate to match closely the capture and use of solar energy by the light reactions. In leaves of C3 plants, the Calvin-cycle acclimates to the constantly changing irradiance of the diurnal light cycle by a combination of light-mediated changes in the activation state of phosphoribulkinase (PRK), ribulosbisphosphate carboxylase oxygenase (Rubisco) and glyceraldehyde 3-phosphate dehydrogenase (Gal3PDH), coarse control, and self-regulating mechanisms involving the levels of ribulose bisphosphate (RuBP) and phosphoglyceric acid (PGA), metabolites associated with these enzymes, fine control. The activation states of the various light regulated enzymes of the Calvin cycle appear to change separately and at different rates in response to light (Figure 5; Geiger and Servaites, 1994a). In all cases the time constant leads to changes over a span of a number of minutes. To balance flux throughout the Calvin cycle under these conditions requires additional control based on emergent properties of the system of control metabolites interacting with a series of responsive enzymes (Figure 6; Geiger and Servaites, 1994b). The resulting self-regulation involves interaction of metabolites not only with a single enzyme but also with other enzymes in the pathway. The increasing levels of complexity in regulation are needed so carbon assimilation can be matched closely with the rate of ATP and NADPH synthesis by the light reactions during the diurnal cycle. As a consequence, the light-activated Calvin cycle enzymes and their associated metabolites such as RuBP and PGA show a characteristic diurnal time course in response to the diurnal light regime, reflecting their role in regulation of the Calvin cycle (Geiger et al., 1991, Servaites et al, 1989a, 1989b, 1991).
Fig. 5. Time courses of photon irradiance, net carbon exchange (NCE) rate, and enzyme activation state during a simulated natural day period. A. Photosynthesis rate (•), photon irradiance (—) maximal photon irradiance was 800 µmol m-2 s-1, B. initial Rubisco activity (•) and total Rubisco activity (m). C.-F. activities of other Calvin cycle enzymes. From Servaites et al., 1991.
Fig. 6. Time courses of RuBP and PGA levels in a sugar beet leaf during a 14-h light regime that simulates a natural day. From Servaites et al., 1989a
Metabolic Flexibility
The result of these two forms of regulation is a condition that can be termed metabolic flexibility which enables the plant to achieve a particular metabolic state in a number of different ways. The concerted action of a number of parameters bring to bear various control processes that can achieve a stable condition, homeostasis, by a number of different combinations. Depending on the starting conditions, adjustment may be made by any one of a number of combinations of enzyme levels, enzyme activation states and metabolite levels. The response of photosynthesis to diurnal irradiance patterns provides an example of metabolic flexibility and of the various factors that need to be considered in programming a diurnal time course of PPF. In such situations the `memory’ of how a stable state was reached affects future regulatory responses.
Consequences of Rapidly Initiated or Gradually Changing Irradiance
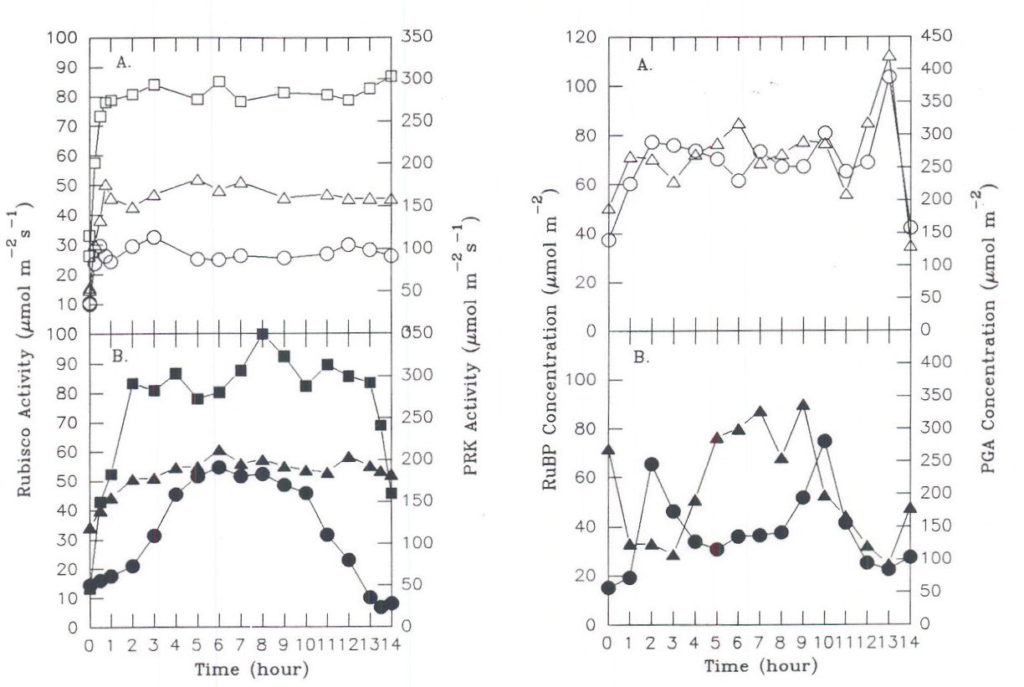
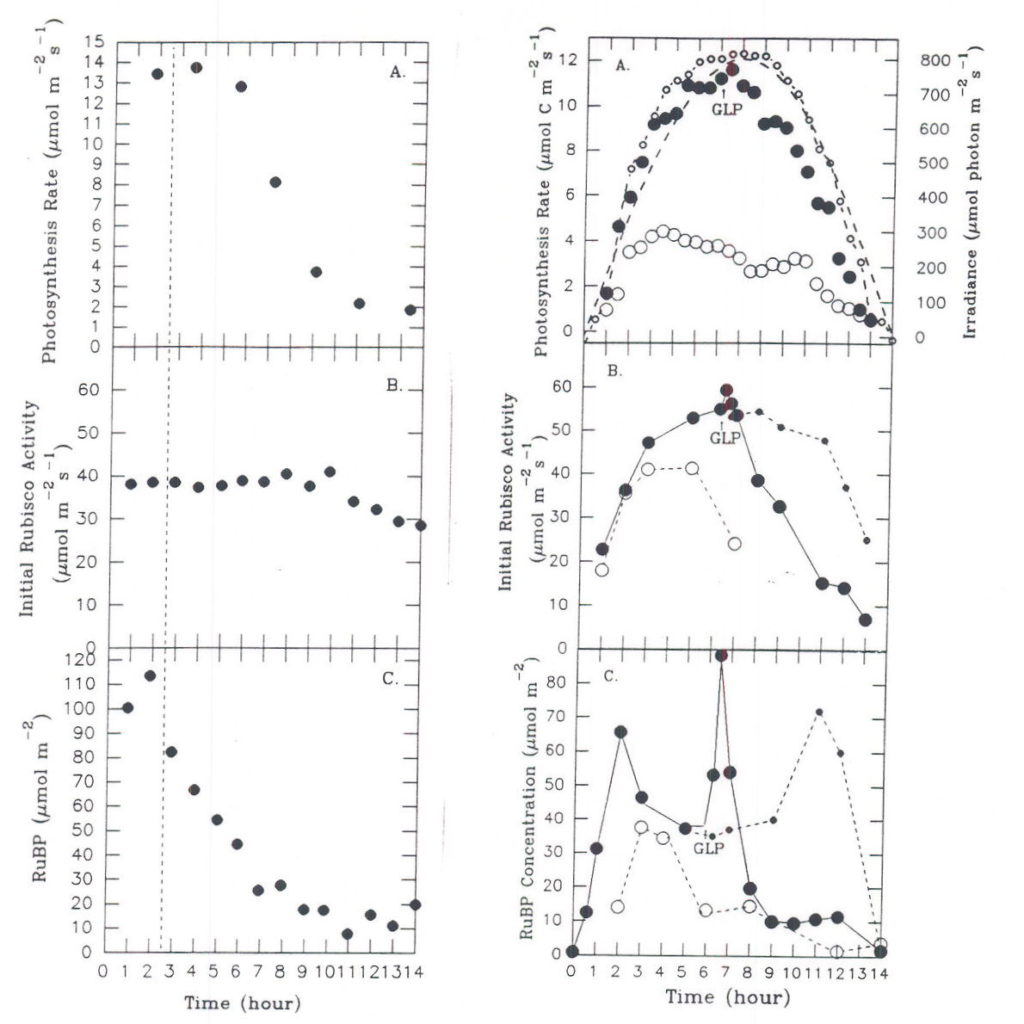
Depending upon initial conditions and the path taken to reach stability, different regulatory elements may assume different degrees of importance. This metabolic flexibility is a result of probabilistic behavior during regulation, in which the path taken to reach stability cannot be described by a unique mathematical solution. The general pattern of response will be conditioned by a number of factors, such as the immediate past history of the plant. This point is well illustrated by observing the response of carbon assimilation in leaves of a sugar beet plant to two different light regimes on successive days. Depending upon how fast illumination reached a maximum at the beginning of the day, different combinations of activation states and associated levels of metabolites were observed for the three enzymes of the assimilatory segment (Servaites et al., 1991; Geiger et al., 1991). Although the leaf maintained similar maximal midday photosynthesis rates under the different light regimes (Figure 7), considerable differences in the degree of Rubisco and PRK activation (Figure 8) and the levels of RuBP and PGA (Figure 9) were observed. Under the gradually increasing light of a diurnal light regime, the midday level of Rubisco activation state was nearly 100% and the RuBP level was about twice Rubisco binding site level. By contrast, when light increased to a maximum rapidly, as often occurs under growth room conditions, the midday level of Rubisco activation state was maintained at only 60% throughout the day, while the RuBP level was nearly twice that observed in the same leaves under gradually increasing light. Regulation by light-mediated enzyme activation was favored under gradually increasing irradiance while metabolite-mediated mechanisms were relatively more important when irradiance increased rapidly. The different forms of regulation achieved similar photosynthesis rates through different combinations of activation states and metabolite levels, an expression of the metabolic flexibility of photosynthesis. As a consequence of the physiological state resulting from metabolic flexibility in regulatory responses, plants may respond differently to stress. The response of photosynthesis to application of glyphosate depends on whether the day began with rapidly initiated irradiance or under gradually changing irradiance (Figure 10). When irradiance was begun rapidly at the start of the day, RuBP level was high, Rubisco activation state was only about 70% (Figure 10 A-C). Under these conditions, inhibition of photosynthesis does not occur until about 4 h after glyphosate is applied, when RuBP level has fallen to a point where its level begins to be a significant factor determining photosynthesis rate (Servaites et al., 1987). In contrast, under gradually changing diurnal irradiance (Figure 10 D-F), Rubisco activation state is full, RuBP is lower and is a significant factor regulating photosynthesis rate. In this case photosynthesis rate begins to decrease along with RuBP leave almost immediately after glyphosate is applied (Figure 10 D-F; Shieh et al., 1991). The physiological state clearly affects the response of photosynthesis to the imposed stress of the inhibition of the shikimate pathway by glyphosate.
A recent study of carbon allocation throughout the day-night cycle revealed that allocation of carbon to the synthesis of sucrose is regulated by an endogenous signal that is adapted to the usual time course of diurnal irradiance (Geiger and Shieh 1994). When the photoperiod begins with rapid onset of high light carbon allocation is changed markedly. Under these conditions, sucrose is synthesized from newly fixed carbon by two biochemical pathways and no starch is produced for several hours. Similarly, if irradiance remains high at the end of the day starch synthesis stops and sucrose synthesis and export nearly double. Clearly, carbon metabolism is changed by departure from the usual daily time course of irradiance. As a result of metabolic flexibility the plant acclimates to the step time course of irradiance often used in growth under artificial light but the metabolic state of the plants are changed.
Fig. 7. Time course of photon irradiance, NCE rate and apparent quantum yield for sugar beet leaves. Data under (A.) rapidly initiated or (B.) gradually changing irradiance. NCE (m, •), photon irradiance (- – – -) and apparent quantum efficiency (——-). Data from Geiger et al., 1991
Fig. 8. (left) Rubisco and PRK activities in sugar beet leaves. Data for plants under (A.) rapidly initiated or (B.) gradually changing irradiance. (m,•) PRK activity; total (_,Ù) or initial (§,¨) Rubisco activity.
Fig. 9. (right) Levels of RuBP and PGA in sugar beet leaves from plants shown in Figure 8. (_,Ù) PGA levels, (§,¨) RuBP levels. From Geiger et al., 1991.
Fig. 10 Time courses of NCE, initial Rubisco activity and RuBP following application of glyphosate to leaves under contrasting light regimes. Data for day of application (•), for second day (m), and for control plants (- – · – -). Vertical dashed line and GLP mark the time of application of glyphosate. A.-C.: Leaf under rapidly initiated irradiance. From Servaites et al., 1987. D.-F.: Leaf under gradually changing irradiance. From Shieh et al., 1991.
RESPONSE TO DIURNAL IRRADIANCE LEVEL
Irradiance Level Affects Photosynthetic Quantum Yield
A measure of successful diurnal regulation of photosynthesis is the ability of the plant to lessen the impact of environmental stresses and so use what light is available efficiently. The result is a general correspondence between carbon flux through the assimilatory segment of the cycle and the course of diurnal irradiance. Recently we conducted a series of studies that dealt with diurnal acclimation of photosynthesis, both in the field and in the laboratory under a light regime that simulated a clear day (Servaites et al., 1989a, 1989b, 1991). Photosynthetic performance was assessed by the apparent quantum yield (Φi), that is, the moles of carbon fixed per mole of incident photons based upon NCE rate and PPF.
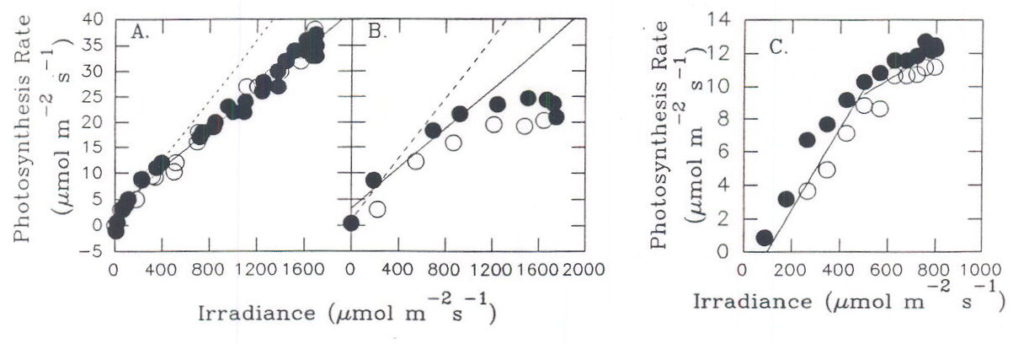
Fig. 11. NCE rate of sugar beet leaves as a function of PPF. A. and B. field-grown plants, on a clear summer day. C. Laboratory-grown plant in moderate light. Data for plants under increasing (•) or decreasing (m) photon irradiance.From Servaites and Geiger, 1994.
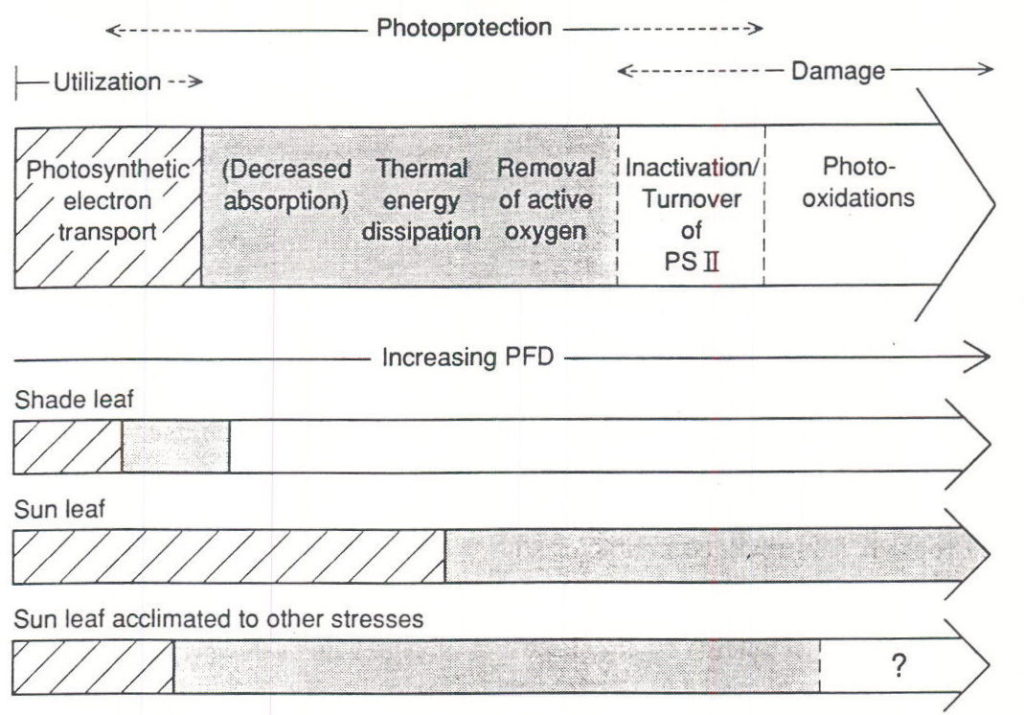
Experiments with field-grown sugar beet plants that were acclimated to growing outdoors showed differences in the maximum diurnal NCE rate attained under the high PPF of summer sunlight (Servaites and Geiger, 1994). In some circumstances, NCE rate increased in nearly direct proportion to PPF throughout the day while in other cases the rates stopped increasing or even decreased with increasing PPF near midday , resulting in a second decrease in Φi. In general, Φi decreased to a new level when irradiance reached about a quarter that of full sunlight (Figures 7, 11), both under moderate light in the laboratory (Figure 11C) and in the field (Figures. 11A,B). More often than not, Φi decreased again under the midday summer sun when irradiance exceeded about half the level of full sunlight (Figure 11B). The initial decreases in Φi at low to moderate PPF likely resulted from photoprotective mechanisms while the midday decrease likely was caused by inactivation or turnover of photosystem II or by photoinhibition (Krause and Weis, 1991; Demmig-Adams and Adams, 1992; Ruban et al., 1993). High temperatures, high leaf-to-air water vapor pressure differences or other environmental factors that show diurnal changes and that can result in stress may have a part in inducing the second change in Φi (references in Bunce 1990). A recent review (Demmig-Adams and Adams, 1992) provides a model representing the responses of the photosynthetic apparatus to increasing levels of PPF. In brief, at first the increase in irradiance can bring about photoprotective responses. If irradiance continues to increase under a certain degree of stress to the photosynthetic apparatus responses may occur that result in greater or lesser damage. It seems reasonable to conclude that the two phases of decrease in Φi correspond generally to these stages of severity in the response to increasing irradiance.
Integrated Daily Light Energy Is Important in Determining Leaf Adaptation to Light
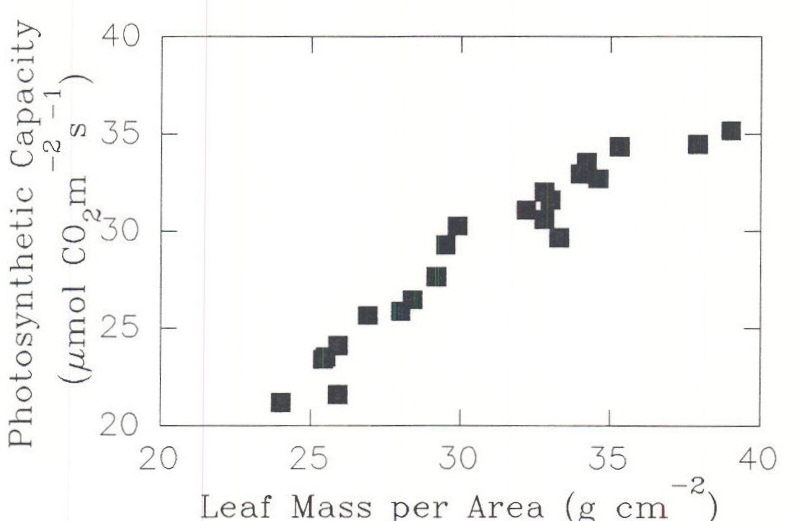
Leaf anatomy and NCE rate per unit dry wt can be modified during leaf expansion to reflect the predominant light conditions (Jurik et al., 1979). Leaf structural and NCE per unit dry wt were similar under environments where the integrated daily light energy was the same even though peak PPF was different (Table 2, Chabot et al., 1979). High total quanta, even at relatively low peak irradiance, produced sun type leaves (Chabot et al. 1979). Total daily incident quanta is a key factor determining leaf thickness, leaf weight per area, and mesophyll cell volume and surface per leaf area. Photosynthetic capacity, the light saturated rate of NCE measured at 25oC and 34 Pa external CO2, could be modified by changes in environmental conditions after leaves had become net exporters of carbon but prior to full expansion (Bunce 1991). Up to a point, photosynthetic capacity increased with leaf mass per area (Figure 12). Photosynthetic capacity is increased by total daily light energy incident on those leaves but not by that incident on other leaves. The net available photoassimilate (supply minus use), increases the leaf mass per area (Figure 13) and so is a key factor in acclimation of leaves to light.
The material presented in this paper present data that suggest some criteria for evaluating growth chamber and greenhouse lighting. Effective lighting should produce plants that perform according to the goals of the project. For example, for physiological studies the plants probably should exhibit morphology and physiology similar to that found in field-grown plants. For other projects the criteria will obviously will be set according to the reason for raising the plants.
TABLE 2 NCE and Leaf Anatomy of Fragaria Virbiniana Under Conditions of Nearly the Same Constant Peak PPF, But Variable Total PPF. Values in the same row followed by the same letter are not significantly different. Data from Chabot et al., 1979.
|
Light Regime* |
|||||
|
High PPF Duration (h) |
3.7 |
7.6 |
15.0 |
6.3 |
15.0 |
|
High PPF (µmol m-2 s-1) |
305 |
305 |
302 |
363 |
371 |
|
Integrated PPF (mol m-2 d-1) |
6.45 |
9.88 |
16.3 |
10.1 |
20 |
|
Leaf Traits |
|||||
|
Maximum NCE (µmol m-2 s-1) (µmol g-2 s-1) |
23.6a 0.50a |
28.2b 0.58a |
29.6b 0.51a |
20.8a 0.47a |
31.6b 0.47a |
|
Thickness (µm) |
113 |
126 |
151 |
138 |
179 |
|
SLW (mg m-2) |
48.6a |
49.9a |
59.5b |
44.4a |
69.0c |
|
Mesoph Cell Vol (mm3 m-2) |
3.9 x 104 |
4.8 x 104 |
6.5 x 104 |
4.9 x 104 |
8.0 x 104 |
|
Ames / A |
10.3a |
11.7a |
15.3b |
11.4a |
16.0b |
*The treatments with less than 15h of high PPF were supplemented with a low PPF of 59 µmol m-2s-1 (0.21 mol m-2hr-1) to provide a 15h photoperiod.
Fig. 12. Photosynthetic capacity as a function of dry mass per unit of area. From Bunce, 1991.
Fig. 13. Increase in mass of leaflets of third trifoliate leaves of soybean compared to dry mass income calculated from the mean 24-h NCE rate for the last 3 days before full leaf expansion. From Bunce, 1991.
REFERENCES
Bunce, J.A. 1990. Afternoon inhibition of photosynthesis in maize. 2. Environmental causes and physiological symptoms. Field Crops Res. 24: 261-271.
Bunce, J.A. 1991. Control of the acclimation of photosynthesis to light and temperature in relation to partitioning of photosynthate in developing soybean leaves. J. Exp. Bot. 42:853-859.
Chabot, B.F., T.W. Jurik, and J.F. Chabot. 1990. Influence of instantaneous and integrated light-flux density on leaf anatomy and photosynthesis. Amer. J. Bot. 66: 940-945.
Demmig-Adams, B., and W.W. Adams, III. 1992. Photoprotection and other responses of plants to high light stress. Annu. Rev. Plant Physiol. Plant Mol. Biol. 43: 599-626.
Geiger, D.R., and J.C. Servaites 1994a. Diurnal regulation of photosynthetic carbon metabolism. Annu. Rev. Plant Physiol. Plant Mol. Biol. 45: (in press).
Geiger, D.R., and J.C. Servaites 1994b. Dynamics of self-regulation of photosynthetic carbon metabolism. Plant Physiol. Biochem. 32:173-183.
Geiger, D.R., and W.J. Shieh. 1994. Photosynthetic carbon metabolism and translocation under an extended light period in wild-type and starch-deficient mutant Nicotiana sylvestris L. Submitted to Plant Physiol.
Geiger, D.R., W.J. Shieh, L.S. Lu, and J.C. Servaites. 1991. Carbon assimilation and leaf water status in sugar beet leaves during a simulated natural light regime. Plant Physiol. 97: 1103-1108.
Jurik, T.W., J.F. Chabot, and B.F. Chabot. 1979. Ontogeny of photosynthetic performance in Frageria virginiana under changing light regimes. Plant Physiol. 63: 542-547.
Kasperbauer, M.J. 1987. Far-red light reflection from green leaves and effects on phytochrome-mediated assimilate partitioning under field conditions. Plant Physiol. 85: 350-354.
Krause, G.H., and E. Weis 1991. Chlorophyll fluorescence and photosynthesis: The basics. Annu. Rev. Plant Physiol. Plant Mol. Biol. 42: 313-349.
Lopez-Juez, E., W.F. Buurmeijer, G.H. Heeringa, R.E. Kendrick, and J.C. Wesselius 1990. Response of light-grown wild-type and long hypocotyl mutant cucumber plants to end-of-day far-red light. Photochem. Photobiol. 52: 143-149.
Ruban, A.V., A.J. Young, P. Horton 1993. Induction of nonphotochemical energy dissipation and absorbance changes in leaves. Plant Physiol. 102: 741-750.
Servaites, J.C., and D.R. Geiger. 1994. Diurnal regulation of photosynthetic carbon metabolism in field-grown sugar beet plants. Photosyn. Res. (submitted).
Servaites, J.C., B.R. Fondy, B. Li, D.R. Geiger 1989a. Sources of carbon for export from spinach leaves throughout the day. Plant Physiol. 90: 1168‑1174.
Servaites, J.C., D.R. Geiger, M.A. Tucci, and B.R. Fondy. 1989b. Leaf carbon metabolism and metabolite levels during a period of sinusoidal light. Plant Physiol. 89: 403‑408.
Servaites, J.C., W.J. Shieh, and D.R. Geiger. 1991. Regulation of photosynthetic carbon reduction cycle by ribulose bisphosphate and phosphoglyceric acid. Plant Physiol. 97: 1115-1121.
Servaites, J.C., M.A. Tucci, and D.R. Geiger. 1987. Glyphosate effects on carbon assimilation, ribulose bisphosphate carboxylase activity, metabolite levels in sugar beet leaves. Plant Physiol. 85: 370‑374.
Shieh, W.J., D.R. Geiger, and J.C. Servaites. 1991. Effect of glyphosate on carbon assimilation and metabolism during a simulated natural day. Plant Physiol. 97: 1109-1114.
Taiz, L., and E. Zeiger. 1991. Plant physiology, Benjamin/Cummings, Redwood City , CA
Geiger, D.R., and G. P. Noname.1994. General lighting requirements for photosynthesis, p 3-18. In: T.W.Tibbitts (ed.). International Lighting in Controlled Environments Workshop, NASA-CP-95-3309.
Copyright © March 1994 NASA [National Aeronautics and Space Administration].
All rights reserved.